Grant Archives — Cancer and Cell Aging Platform
Title: Programmed death-ligand 1 promoting the epithelial-to-mesenchymal transition implies novel therapeutic potentials in triple-negative breast cancer
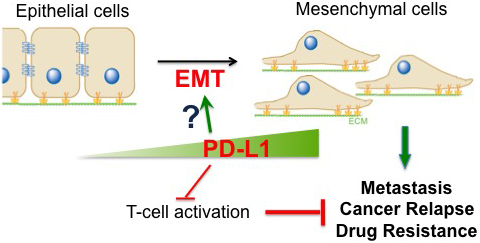
The many roles of PD-L1. Although PD-L1 is well-known to inhibit T cell function, the research team's data highlight important roles for PD-L1 in cancer cells that drive tumor formation, drug resistance and metastasis.
Investigative team: Kun Ling, Ph.D., Haidong Dong, M.D., Ph.D., Liewei Wang, M.D., Ph.D.
Central hypothesis: Triple-negative breast cancer (TNBC) is an important clinical challenge due to its poor prognosis and lack of response to current therapies. Recently, the investigative team and others observed that programmed death-ligand 1 (PD-L1), an immune-checkpoint molecule, is expressed in TNBC cells and is involved in drug resistance of breast cancer cells. This suggests that PD-L1 has immune-checkpoint independent functions.
Based on its preliminary studies, the team is testing the hypothesis that PD-L1 contributes to TNBC progression by promoting epithelial-to-mesenchymal transition (EMT), which contributes to cancer metastasis, relapse and drug resistance.
Potential outcomes and advances: The research team is working to define the novel function for PD-L1 in tumor metastasis and drug resistance of TNBC cells. New drug targets and therapeutic strategies may be identified based on the resulting new knowledge of the molecular mechanisms underlying PD-L1-dependent EMT and the cross-talk between PD-L1 and other pathways within TNBC cells.
Back to topTitle: Role of PLEKHA7-mediated miRNA regulation in KRAS-induced lung cancer progression
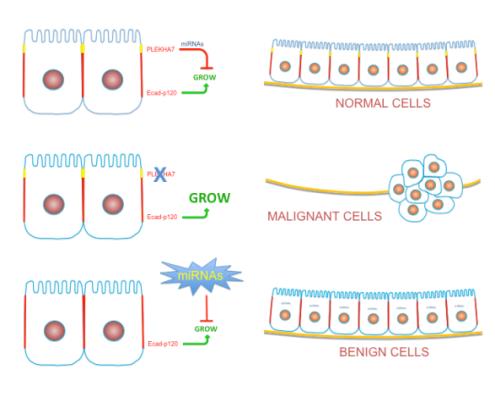
An adhesion complex containing PLEKHA7 engages miRNAs to suppress growth signals in normal epithelia. The research team proposes that restoring affected miRNAs will revert the malignant phenotype of cells lacking PLEKHA7 function.
Investigative team: Panagiotis Z. Anastasiadis, Ph.D., Alan P. Fields, Ph.D., Andras Khoor, M.D., Ph.D., E. Aubrey Thompson, Ph.D.
Central hypothesis: Lung cancer is the leading cause of cancer death in the U.S. A key event in lung cancer progression is the transition from a benign adenoma to a malignant adenocarcinoma. This transition is marked by dramatic changes in cell morphology and gain of aggressive behaviors such as increased tumor growth and invasive properties.
The investigators hypothesize that the loss of a protein called PLEKHA7 and the subsequent deregulation of small RNA molecules called microRNAs (miRNAs) drive the transition from a lung adenoma to an adenocarcinoma. They also propose that restoring the normal levels of miRNAs in cancer cells can revert these cells back to a less aggressive state.
Potential outcomes and advances: This study will elucidate the role of PLEKHA7, and the miRNAs it regulates, in the transition of a lung adenoma to a carcinoma. PLEKHA7-mediated regulation of miRNAs provides an unexpected mechanism of growth regulation and a novel therapeutic target. Successful completion of this research could lead to novel therapies for lung cancer.
Back to topTitle: AR-RNA hybrid molecules promote prostate cancer progression

Enhancer RNAs in prostate cancer have the potential to promote resistance to anti-androgens. Identification of these eRNAs could identify new therapeutic targets to impair the androgen resistance mechanism and improve therapy.
Investigative team: Haojie Huang, Ph.D., Stephen C. Ekker, Ph.D., Georges Mer, Ph.D., R. Jeffrey Karnes, M.D., Jun Zhang, M.D.
Central hypothesis: More than 250,000 people worldwide die of castration-resistant prostate cancer (CRPC) each year. Although tumors are initially sensitive to androgen withdrawal hormone therapy, abnormal reactivation of the androgen receptor (AR) is a central mechanism that contributes to therapy resistance and the generation of CRPC. The investigators hypothesize that unique RNA molecules bind to the AR, regulate its activity and contribute to the development of CRPC.
Potential outcomes and advances: The focus of this grant is to uncover how AR-RNA hybrid molecules regulate AR-dependent genes. The investigative team expects that the findings from the proposed experiments will not only shed new light on the etiology of CRPC, but also identify novel targets for the development of new therapeutics to treat this lethal disease.
Back to topTitle: Identifying and targeting epigenetic defects in cancer
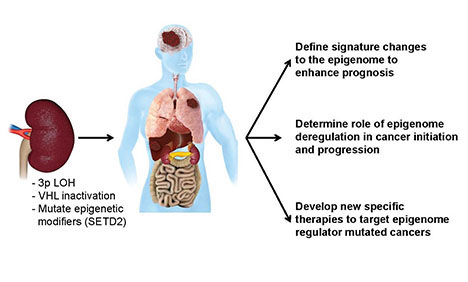
Identifying and targeting epigenetic defects in human cancers
Investigative team: Keith D. Robertson, Ph.D., Thai H. Ho, M.D., Ph.D., Nathalie Meurice, Ph.D., Douglas Lake, Ph.D., Mia D. Champion, Ph.D., Melissa L. Stanton, M.D., Jeanette E. Eckel Passow, Ph.D., Alexander S. Parker, Ph.D.
Central hypothesis: Clear cell renal cancer (ccRCC) is among the 10 leading causes of cancer death in the U.S., with approximately 15,000 deaths annually. Genes that regulate the epigenome are targeted for mutations in more than 50 percent of ccRCC cases, making this group of genes the most frequently mutated in ccRCC and other types of tumors. The investigators are examining the contribution of one of these mutated epigenome modifiers, SETD2, to determine the mechanism by which it contributes to the generation of ccRCC.
Potential outcomes and advances: The project is expected to yield new kidney cancer treatments that can be personalized to patients with SETD2 mutations and will also provide fundamental new insight into how these mutations drive cancers in other tissue types.
Back to topTitle: Intercellular cross-talk via extracellular vesicles and their impact in liver diseases

Exomes are extracellular vesicles (EV) that can be secreted in a bidirectional manner from polarized epithelial cells. The generation, vesicle content and impact of the EV on surrounding cells could drive normal and abnormal cellular function of surrounding cells.
Investigative team: Nicholas F. LaRusso, M.D., Edward B. Leof, Ph.D.
Central hypothesis: This grant focuses on understanding the pathology of liver diseases by analyzing how damaged liver cells communicate with each other to promote disease progression and aggression. Nano-sized sacs released from cellular plasma membrane (extracellular vesicles) are important mediators that shuttle information from one cell to another.
Distinct intracellular pathways and molecules direct the targeted, domain-specific (that is, apical versus basolateral) biogenesis and release of subpopulations of exosomes containing different cargo that differentially influence the phenotype of target cells and modify disease.
Potential outcomes and advances: Identifying specific subpopulations of extracellular vesicles released from distinct cellular locations, their cell targets and the delivery of their distinct content to these target cells in the context of cholangiocyte-related liver diseases (cholangiopathies) will enhance not only the understanding of the diseases but also the ability to treat them.
Back to topTitle: Cancer flips a metabolic switch to grow
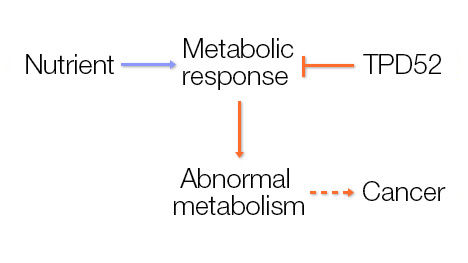
Normal cells respond to nutrients in their environment to regulate their metabolism and need for energy. Cancer cells, however, harness these pathways to grow in an unchecked and metabolically advantaged manner. Discerning the ways in which these metabolic pathways are altered will likely identify key molecules that could be targeted therapeutically in cancer.
Investigative team: Zhenkun Lou, Ph.D., Taro Hitosugi, Ph.D.
Central hypothesis: In women, breast cancer is the most common cancer and the second-leading cause of cancer death. A better understanding of the development of breast cancer would help prevent and treat this disease. Recent studies suggest that cancer cells and noncancerous cells differ in nutrient usage and energy generation. The investigators hypothesize that changes in energy metabolism are important for the development of cancer and that uncovering the molecular pathways involved will identify new avenues for treatment.
Potential outcomes and advances: The project is expected to provide fundamental new insights into a key mechanism in driving breast cancer growth and lead to new personalized treatments for people with breast cancer who also have metabolic deregulation. Moreover, since the metabolic pathway under investigation appears to be deregulated in other cancers, this work has the potential to help people with other types of tumors.
Back to topTitle: Decoding the modified epigenome in brain cancer
Investigative team: Jann N. Sarkaria, M.D., David J. Daniels, M.D., Ph.D.
Central hypothesis: Glioblastoma multiforme (GBM) is the most aggressive type of primary brain tumor in adults and children. Recently, somatic mutations in the H3F3A gene have been detected in 60 percent of high-grade pediatric GBM cases. It is unknown how H3F3A mutation promotes tumorigenesis. In this project, the investigative team is testing the hypothesis that the H3F3A mutation dominantly reprograms the epigenome, resulting in aberrant gene expression, and thereby promotes tumorigenesis.
Potential outcomes and advances: The proposed investigation will uncover basic mechanisms by which mutations in genes that regulate the epigenome can promote cancer. This research has the potential to discover new genes in GBM that are deregulated by H3F3A mutation, and thus represent new therapeutic targets to treat this devastating disease.
Back to top