-

Investigating cellular pathways as druggable targets
Research in Dr. Caulfield's lab cuts across all areas of investigation at Mayo Clinic. Primarily through using in silico technologies, the lab employs techniques that aid with structural biology, cell biology, enzymology, protein engineering and nanodesign implementation.
-
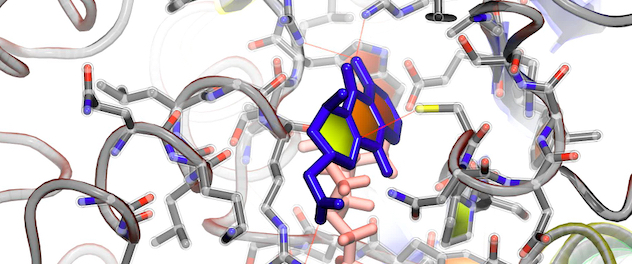
Novel quantum docking technology for drug design
Molecular positioning of a lead molecule for inhibition of the cancer target DNA methyltransferase 3 beta (DNMT3B) enzyme is shown as a result from quantum mechanics-based scoring while under the Maxwell's demon molecular dynamics (MdMD) algorithm called qDockMdMD. The lab's MdMD program is derived from a thought experiment proposed by James Clerk Maxwell, a 19th century physicist.
-
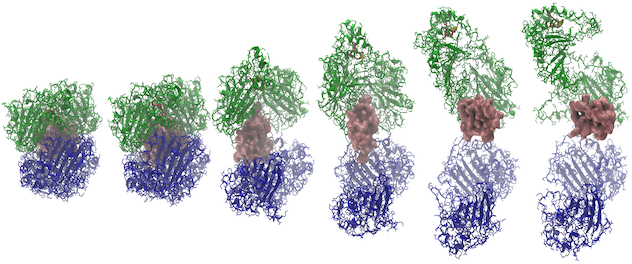
Insulin-degrading enzyme capture and release of insulin
Using the MdMD algorithm to complete simulations that rapidly sample dynamic conformational changes in the protein, we are able to determine how insulin-degrading enzyme (IDE) can degrade insulin and develop IDE inhibitors to alter insulin pathways.
Overview
Mayo Clinic's Drug Discovery, Design and Optimization for Novel Therapeutics Laboratory is led by Thomas Caulfield, Ph.D., and focuses on the molecular interactions and driving forces that enable biomolecular activities to be studied at the atomic level. This atomic-level knowledge guides investigation of these interactions to alter cellular pathways.
A crucial step in druggability and drug engineering is understanding the cellular target's behavior. Typically, our cellular targets are proteins composed of folded amino acid chains in complexes. The targets can be drawn from multiple data sources, such as cryogenic electron microscopy, also known as cryo-EM, X-ray crystallography and kinetics studies. This then enables us to dynamically model processes underlying change over time. This time factor is crucial to capturing the fundamental behavior of the biomolecules. The lab also studies ribonucleic complexes and nanoparticle constructs.
Drug engineering aims to accelerate the benchtop phase of drug development, since our lab can rapidly test tens of thousands to many millions of combinations of potential drug molecules rapidly. This saves our collaborators time and money, allowing us to propose intelligent hypotheses underlying the structure-function behavior we observe.
The lab primarily applies our in silico approaches toward neurodegeneration, cancer biology and metabolic disorders. Dr. Caulfield and his team engage in collaborative endeavors with Mayo Clinic colleagues from areas such as neuroscience, cancer biology, neurosurgery, quantitative health sciences, clinical genomics, biochemistry and molecular biology, hematology-oncology, gastroenterology, infectious disease, and cardiology.
In some disease processes, the delicate balance between production, activation and inhibition of the proteins, enzymes, ribonucleic acids and other biomolecules is disturbed, leading to disease progression. These various biomolecules then become our lab's targets for dissecting biomolecular behavior using simulations and in silico methods coupled with experimental feedback.
Related to this, the Drug Discovery, Design and Optimization for Novel Therapeutics Lab works with the Center for Individualized Medicine to aid in dissection of variants in disease related to genomics and personalized medicine. Dr. Caulfield works with the Precision Cancer Therapeutics Program within the Center for Individualized Medicine to make novel anti-cancer agents in conjunction with participating laboratories.
The lab studies these targets for changes in functional behavior that comes as a consequence of structural changes in their 3D shape that occur over a 4D set of properties. This time propagation effect is observed in proteins when introducing mutagenic amino acids that deviate from the wild-type sequence. The outcome of the genetic variation, called a variant, can be an overactive or underactive enzyme or protein. This protein is part of a cellular pathway that can cause large changes to the system (organs, tissues and pathologies) resulting in cancers, for example. Part of the lab's mission is to find agents or compounds that can have a restorative effect on the pathway by either blocking or accelerating the protein or enzyme behavior to counter the effect from the variant.
Identification of altered interactions provides potentially useful therapeutic intervention gateways to probe, which may allow us to search for new candidate drugs suitable for preclinical studies. Ultimately, this may result in clinically relevant drugs for trials.
By studying the interactions between these targets and inhibitors or activators, our lab aspires to continue to design better agents for use in a wide range of medicinal research areas, including neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis (ALS).
Broad research focus
Fundamentally, Dr. Caulfield and his colleagues are working to:
- Structurally determine the mechanistic drivers underlying diseases.
- Investigate the pathogenicity or protective effects of genetic variants for structural basis of missense mutation effect on translated protein products, including a protein informatics platform. This is in conjunction with Mayo Clinic's Center for Individualized Medicine.
- AI-driven drug discovery.
- Develop novel small molecule, peptide-based or biologics therapeutics.
Individual focus areas
- Tau structural studies (from experimental to AI).
- Alzheimer's disease and A-beta protein (AI discovery).
- Variants of uncertain significance categorizing pathogenicity using AI.
- Methods development: quantum-based adaptive docking using Maxwell's demon molecular dynamics (MdMD).
- Ribosomopathies, frameshifting and ribosomal quality control mechanisms.
- SARS-CoV-2 (COVID-19) multidrug high-throughput screening in collaboration with Harvard University and the University of California (see related publication).
To accomplish these goals, Dr. Caulfield's research team brings together multidisciplinary and translational approaches. Through combining structural, molecular, cellular and biochemical outputs from various laboratories, complex decisions are driven by context integrated from multiple data sources. Data sources include high-content imaging, X-ray crystallography, cryo-EM data, and dose response curves (DRCs) and kill assays for EC50s/IC50s. The resulting data fusion assimilates all of these data into various adaptive learning algorithms.
The lab's multi-omics approach brings aboard organ-on-a-chip and animal modeling through collaborations that feed back into both ligand-based and structure-based drug design.
Functional studies with collaborators allow us to do virtual-to-actual screening in rapid rotation for delivery of customizable high-confidence libraries for testing hypotheses and mechanistic validation. Our 3D quantitative structure-activity relationship (3D-QSAR) methodology when combined with our machine learning and data layering allows for transformative de novo drug generation that takes labs from hits to leads and leads through optimization. Further progress continues before and during preclinical stages by the formation of early-stage companies (series A-C) and includes the drug development of pharmacokinetic-pharmacodynamic (Pk-Pd) models and absorption, distribution, metabolism and excretion toxicity (ADMET).