Thermodynamic Behavior of Molecules Relevant to Pancreatitis and Organ Failure
Dr. Singh's research team in his Pancreatitis and Acute Outcomes Research Lab is investigating the biological behavior, specifically the thermodynamics of reactions and interactions, of molecules with a crucial role in pancreatitis. These include bile acids, lipids and the proteins that interact with them.
The lab's team has provided thermodynamic evidence that lipids and bile acids can be independent signaling molecules in aqueous environments, and the magnitude of their signaling and consequent biological responses are dependent on core temperature. Such behavior involves micellar to monomer transition, and multiple aspects can be studied using isothermal titration calorimetry, verifying the aqueous behavior of these molecules with complementary physical chemistry methods.
Effect of temperature change on micellar behavior of sodium taurocholate
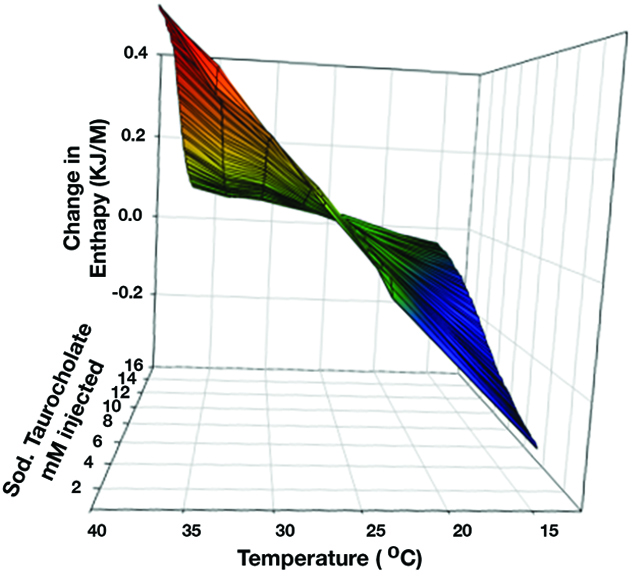
How temperature change affects the micellar behavior of the common bile salt sodium taurocholate in phosphate-buffered saline.
As shown in the graph, there is no change in enthalpy at 25 C over the 0-16 mM concentration range of sodium taurocholate (STC) supporting cessation of micellar breakdown at this temperature. This dramatic alteration in STC behavior by the mere 12 C from 37 C to 25 C lends support to our discovery that local hypothermia of the pancreas can treat pancreatitis. This work was published in the Gastroenterology Journal, 2019.
Agents that cause or worsen pancreatitis are active signaling molecules that induce local and systemic damage. Such agents include NEFAs and bile acids with hydrophobic domains, which greatly impact their behavior in an aqueous environment. This affects their interactions with cells and the biological responses they elicit. Therefore, Dr. Singh's lab is studying the micellar to monomer transition of these agents, using physical chemistry to understand the biological responses induced by these agents.
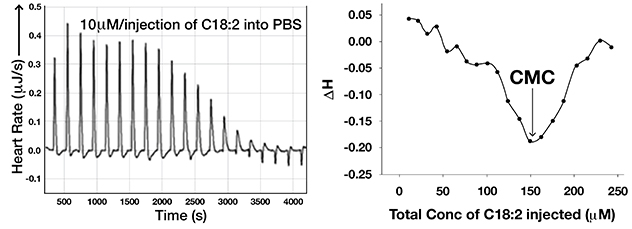 Linoleic acid signaling as a monomer at specific concentrations
Linoleic acid signaling as a monomer at specific concentrations
Raw data from isothermal titration calorimetry used to study micellar breakdown of linoleic acid (C18:2) in phosphate buffered saline (PBS), left side. Critical micellar concentration (CMC) deduced from this experiment, right side. This shows that linoleic acid can signal as a monomer at concentrations less than 150 µM in PBS.