Research Registration Application
The Research Registration Application is a Mayo Clinic-designed app supporting Mayo's role as a registration and randomization center for clinical trials. It can be used to:
- Validate patient enrollment
- Collect patient demography and accrual data
- Generate user reports on enrollment data
- Establish controls around the patient enrollment process
The Research Registration Application provides a mechanism for study staff, registration specialists and other key study personnel to access and input patient registration information for clinical trials initiated by Mayo Clinic.
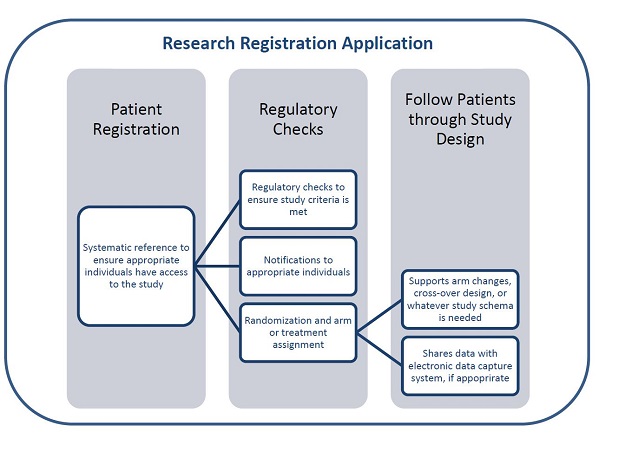
The Research Registration Application can be used to register patients, perform regulatory checks and follow patients through studies.
Access
Authorized users can access the Research Registration Application on the Research Registration website.
If you are at a site participating in a clinical trial that is utilizing the Research Registration system, please follow these steps to receive access:
- Work with your study-specific Mayo Clinic contact to ensure that your site has been accepted to participate in the specific study of interest and that regulatory documentation has been completed, such as protocol-specific training, Good Clinical Practice training certificates, delegation of authority, the Food and Drug Administration's 1572 form, licensure and so on.
- Complete an interactive training course on the use of the Research Registration Application.
- Download, print, complete and sign the Attestation of Training form (Word document) and email a scanned version of the form to ResearchSiteManagement@mayo.edu.
The Office of Clinical Trials site management team will contact you after providing access.
Contact
To learn more about Mayo Clinic's Research Registration Application, email ResearchSiteManagement@mayo.edu or call
507-284-4130.