概述
多发性硬化
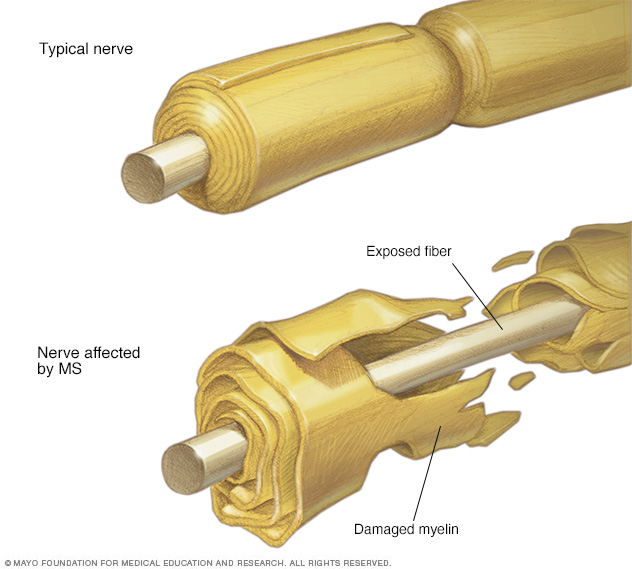
多发性硬化
多发性硬化症患者的神经纤维保护层(髓磷脂)先是受损,最终可能被破坏。根据神经损伤的部位,MS 可能影响视力、感觉、协调、运动以及排尿或排便。
多发性硬化症(MS)是一种可能导致大脑和脊髓(中枢神经系统)失能的疾病。
MS 患者的免疫系统会攻击包覆神经纤维的保护鞘(髓磷脂),导致大脑无法与身体其他部分进行沟通。最终,这种疾病可能导致神经纤维永久性损伤或退化。
MS 的体征和症状存在患者差异,具体取决于中枢神经系统中神经纤维损伤的部位和严重程度。一些重度 MS 患者可能无法独立行走或根本无法行走。其他个体可能经历长期缓解,而不会出现任何新的症状,这取决于患者的 MS 类型。
多发性硬化症尚无治愈方法。但是,治疗有助于加快恢复速度,改变这种疾病的病程并控制症状。
症状
髓磷脂损伤和神经系统
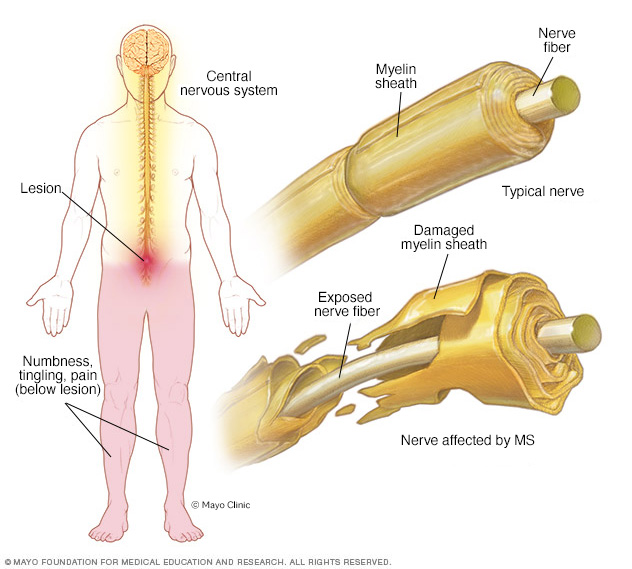
髓磷脂损伤和神经系统
患多发性硬化时,中枢神经系统中的神经纤维保护层(髓磷脂)受损。这会造成病变,进而导致身体部分麻木、疼痛或刺痛等症状,具体取决于中枢神经系统的位置。
多发性硬化症的体征和症状可能在每个人之间和整个疾病过程中会有很大差异,这取决于受影响神经纤维的位置。
常见症状包括:
- 一个或多个肢体有麻木感或无力,每次通常是身体其中一侧发作。
- 麻刺感
- 颈部活动时有触电的感觉,尤其是颈部向前弯曲时(莱尔米特征)
- 缺乏协调性
- 步态不稳或无法行走
- 视力部分或完全丧失,通常一次只发生在一只眼睛上,眼球运动时常伴有疼痛
- 长时间复视
- 视物模糊
- 眩晕
- 性功能、肠道功能和膀胱功能问题
- 疲劳
- 言语不清
- 认知问题
- 情绪障碍
何时就诊
See a doctor if you experience any of the above symptoms for unknown reasons.
病程
大多数多发性硬化症患者都会经历一个复发缓解的病程。他们会经历持续数天或数周的新症状或复发症状,但这些症状通常会部分或完全好转。复发后是一个病情缓解的平静期,这段时间可持续数月甚至数年之久。
体温小幅增长会暂时加重多发性硬化症的体征和症状,但这不属于真正的疾病复发而是伪复发。
从发病起,至少 20% 至 40% 的复发缓解型多发性硬化症患者最终会在 10 到 20 年内形成稳定发展的症状,中间存在或不存在缓解期。这种情况称为继发进展型多发性硬化症。
症状恶化通常包括行动和步态问题。对于继发进展型 MS 患者,其病情进展速度具有很大的差异性。
有些 MS 患者的发病体征和症状是逐渐产生的,并保持稳定发展,无复发,称为原发进展型 MS。
病因
多发性硬化症的病因不明。它被认为是一种免疫介导疾病,患此疾病后,身体的免疫系统会攻击自己的组织。患有 MS 时,这种免疫系统障碍会破坏包裹并保护大脑和脊髓神经纤维的脂肪物质(髓磷脂)。
髓磷脂相当于电线上的绝缘涂层。如果保护性髓磷脂受损而露出神经纤维,沿神经纤维传递的信息可能减慢或受阻。
目前还不清楚为什么有些人会患 MS,而另一些人不会。基因和环境因素似乎是造成这一病症的原因。
风险因素
这些因素可能会使多发性硬化症的患病风险增加:
- 年龄。MS 可发生于任何年龄段,但通常见于 20 岁和 40 岁左右。但是年龄较小者和年龄较大者都会患病。
- 性别。女性患复发 - 缓解型 MS 的几率比男性高 2 至 3 倍。
- 家族史。如果您的父母或兄弟姐妹中有一人患 MS,您患这种疾病的风险就更高。
- 某些感染。多种病毒与 MS 有关,包括引起传染性单核细胞增多症的艾巴氏病毒。
- 种族。白人(尤其是北欧血统的白人)患 MS 的风险最高。亚裔、非裔或美洲原住民的风险最低。最近的一项研究表明,患有多发性硬化症的黑人和西班牙裔年轻人的数量可能比以前认为的要多。
- 气候。MS 在温带气候国家更为常见,包括加拿大、美国北部、新西兰、澳大利亚东南部和欧洲。出生月份也可能影响患多发性硬化症的几率,因为母亲怀孕时晒太阳似乎会减少孩子以后患多发性硬化症的几率。
- 维生素 D。 维生素 D 含量低、阳光照射少,患 MS 的风险更大。
- 基因。已发现染色体 6p21 上的一个基因与多发性硬化症有关。
- 肥胖症。已发现女性的肥胖症与多发性硬化症存在关联。对女性儿童期和青少年期的肥胖症而言尤其如此。
- 某些自身免疫性疾病。如果您患有其他自身免疫性疾病,如甲状腺疾病、恶性贫血、银屑病、1 型糖尿病或炎性肠病,则患 MS 的风险稍高。
- 抽烟。出现可能指示 MS 的初始症状的抽烟者比不抽烟者更可能出现第二个症状,即确证复发-缓解型 MS。
并发症
多发性硬化症患者还可能出现:
- 肌肉僵硬或痉挛
- 严重虚弱或瘫痪(一般在腿部)
- 膀胱、肠道或性功能出现问题
- 认知问题,如健忘或找词困难
- 抑郁症、焦虑症或情绪波动等情绪问题
- 癫痫发作,但非常罕见。
Dec. 24, 2022
- What is multiple sclerosis? National Multiple Sclerosis Society. https://www.nationalmssociety.org/What-is-MS. Accessed June 2, 2022.
- Daroff RB, et al. Multiple sclerosis and other inflammatory demyelinating diseases of the central nervous system. In: Bradley's Neurology in Clinical Practice. 7th ed. Philadelphia, Pa.: Elsevier Saunders; 2012. https://www.clinicalkey.com. Accessed June 2, 2022.
- Ferri FF. Multiple sclerosis. In: Ferri's Clinical Advisor 2019. Philadelphia, Pa.: Elsevier; 2019. https://www.clinicalkey.com. Accessed June 2, 2022.
- Olek MJ. Clinical presentation, course, and prognosis of multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Wingerchuk DM (expert opinion). Mayo Clinic, Phoenix/Scottsdale, Ariz. Jan. 21, 2019.
- Ciccarelli O. Multiple sclerosis in 2018: New therapies and biomarkers. The Lancet. 2019; doi: 10.1016/S14744422 (18)30455-1.
- Keegan BM. Therapeutic decision making in a new drug era in multiple sclerosis. Seminars in Neurology. 2013; doi:10.1055/s0033-1345709.
- Goldman L, et al., eds. Multiple sclerosis and demyelinating conditions of the central nervous system. In: Goldman-Cecil Medicine. 25th ed. Philadelphia, Pa.: Saunders Elsevier; 2016. https://www.clinicalkey.com. Accessed Jun. 2, 2022.
- Lotze TE. Pathogenesis, clinical features, and diagnosis of pediatric multiple sclerosis. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Kantarci OH, et al. Novel immunomodulatory approaches for the management of multiple sclerosis. Clinical Pharmacology & Therapeutics. 2014; doi:10.1038/clpt.2013.196.
- Olek MJ. Disease-modifying treatment of relapsing-remitting multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Olek MJ, et al. Treatment of acute exacerbations of multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Wingerchuk DM. Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clinic Proceedings. 2014; doi:10.1016/j.mayocp.2013.11.002.
- Pizzorno JE, et al. Multiple sclerosis. In: Textbook of Natural Medicine. 4th ed. St. Louis, Mo.: Churchill Livingstone Elsevier; 2013. https://www.clinicalkey.com. Accessed June 2, 2022.
- Olek MJ, et al. Evaluation and diagnosis of multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Gaetani L, et al. 2017 revisions of McDonald criteria shorten the time to diagnosis of multiple sclerosis in clinically isolated syndromes. Journal of Neurology. 2018;265:2684.
- http://onlinelibrary.wiley.com/doi/10.1002/ana.22366.
- Olek MJ, et al. Pathogenesis and epidemiology of multiple sclerosis.
- Ingram G, et al. Cannabis and multiple sclerosis. Practical Neurology. 2019; doi:10.1136/practneurol-2018-002137.
- Olek MJ, et al. Symptom management of multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Yadav Y, et al. Summary of evidence-based guideline: Complementary and alternative medicine in multiple sclerosis. Neurology. 2014; doi: 10.1212/WNL.0000000000000250.
- Nimmagadda R. Allscripts EPSi. Mayo Clinic. April 22, 2022.
- National MS Society. Network of Pediatric MS Centers. https://www.nationalmssociety.org/What-is-MS/Who-Gets-MS/Pediatric-MS/Care-for-Pediatric-MS. Accessed June 2, 2022.
- Rodriguez M. Plasmapheresis in acute episodes of fulminant CNS inflammatory demyelination. Neurology. 1993; doi:10.1212/wnl.43.6.1100.
- Deb C. CD8+ T cells cause disability and axon loss in a mouse model of multiple sclerosis. PLoS One. 2010; doi:101371/journal.pone.0012478.
- FDA approves new drug to treat multiple sclerosis. U.S. Food & Drug Administration. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm549325.htm. Accessed June 1, 2022.
- Keegan BM (expert opinion). Mayo Clinic, Rochester, Minn. January 15, 2019.
- FDA approves new oral drug to treat multiple sclerosis. U.S. Food and Drug Administration. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm634469.htm. Accessed June 2, 2022.
- Kappos L, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomized, phase 3 study. The Lancet. 2018; doi: 10.1016/S0140-6736(18)30475-6.
- Marin Collazo IV (expert opinion). Mayo Clinic, Rochester, Minn. April 2, 2019.
- AskMayoExpert. Multiple sclerosis. Mayo Clinic; 2020.
- AskMayoExpert. Medication monitoring guidelines. Mayo Clinic; 2020.
- Vumerity. National MS Society. https://www.nationalmssociety.org/Treating-MS/Medications/Vumerity. Accessed March 16, 2020.
- Gianfrancesco M, et al. Obesity during childhood and adolescence increases susceptibility to multiple sclerosis after accounting for established genetic and environmental risk factors. Obesity Research and Clinical Practice. 2014; doi.org/10.1016/j.orcp.2014.01.002.
- Pantavou KG, et al. Season of birth and multiple sclerosis: A systematic review and multivariate meta-analysis. Journal of Neurology. 2020; doi:10.1007/s00415019-09346-5.
- Cifu DX, et al., eds. Multiple sclerosis. In Braddom's Physical Medicine and Rehabilitation. 6th ed. Elsevier; 2021 https://www.clinicalkey.com. Accessed Jun. 2, 2022.
- Langer-Gould AM, et al. Racial and ethnic disparities in multiple sclerosis prevalence. Neurology. 2022; doi:10.1212/WNL.0000000000200151.
- Kasper LH, et al. Immunomodulatory activity of interferon-beta. Annals of Clinical and Translational Neurology. 2014; doi:10.1002/acn3.84.
- Goldschmidt CH, et al. Re-evaluating the use of IFN-B and relapsing multiple sclerosis: Safety, efficacy and place in therapy. Degenerative Neurological and Neuromuscular Disease. 2020; doi:10.2147/DNND.S224912.
- Kieseie BC. The mechanism of action of interferon-B in relapsing multiple sclerosis. Central Nervous System Drugs. 2011; doi:10.1007/s10067-008-0972-3.
- Betaseron. Bayer AG; 1993. www.bayer.com. Accessed Jun. 1, 2022.
- Hauser SL, et al. Ofatumumab versus teriflunomide in multiple sclerosis. The New England Journal of Medicine. 2020; doi:10.1056/NEJMoa1917246.
- Kesimpta. Novartis; 2020. www.novartis.com. Accessed Jun. 1, 2022.
- Marin Collazo V (expert opinion). Mayo Clinic. June 13, 2020.
- Olek MJ. Treatment of progressive multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed Jun. 2, 2022.
- Wingerchuk DM, et al. Disease modifying therapies for relapsing multiple sclerosis. British Medical Journal. 2016; doi:10.1136/bmj.i3518.
- Saadeh RS, et al. CSF kappa free light chains: Cutoff validation for diagnosing multiple sclerosis. Mayo Clinic Proceedings. 2022; doi:10.1016/j.mayocp.2021.09.014.
- Goldschmidt C, et al. Advances in the treatment of multiple sclerosis. Neurologic Clinics. 2021; doi:10.1016/j.ncl.2020.09.002.
- Bafiertam. Banner Life Sciences LLC; 2013. www.bannerls.com. Accessed Jun. 1, 2022.
- Baliertam delayed release capsule. Banner Life Sciences LLC; 2013. www.bannerls.com. Accessed Jun. 1, 2022.
- Oral ponesimod versus teriflunomide in relapsing multiple sclerosis (OPTIMUM). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02425644. Accessed Jun. 2, 2022.
- Ponvory. Janssen Pharmaceuticals; 2021. www.janssen.com. Accessed Jun. 1, 2022.
- Torke S, et al. Inhibition of Bruton's tyrosine kinase as a novel therapeutic approach in multiple sclerosis. Expert Opinion on Investigational Drugs. 2020.
- Nash RA, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsing-remitting multiple sclerosis (HALT-MS): A 3-year interim report. Journal of the American Medical Association Neurology. 2015; doi:10.1001/jamaneurol.2014.3780.
- Reston, et al. Autologous hematopoietic cell transplantation for multiple sclerosis: A systematic review. Multiple Sclerosis. 2011; doi:10,1177/1352458510383609.
- Petrou P, et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain. 2020; doi:10.1093/brain/awaa333.
- Liang J, et al. Allogenic mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Multiple Sclerosis. 2009; doi:10.1177/1352458509104590.
- Wingerchuk DM, et al. Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clinic Proceedings. 2014; doi:101016/j.mayocp.2013.11.002.
- Multiple sclerosis information page. National institute of neurological disorders and stroke. https://www.ninds.nih.gov/Disorders/All-Disorders/Multiple-Sclerosis-Information-Page. Accessed Jun. 2, 2022.
- Wingerchuk DM, et al. Disease modifying therapies for relapsing multiple sclerosis. British Medical Journal. 2016; doi:10.1136/bmj.i3518.
- Sadovnick AD. Genetic background of multiple sclerosis. Autoimmunity Reviews. 2012; doi:10.1016/j.autrev.2011.05.007.