Uncovering the Signaling Events Underlying the Development and Progression of Pancreatic Cancer
Pancreatic ductal adenocarcinoma is one of the most aggressive types of cancer. In the absence of early detection methods and effective treatment, Dr. Storz's Tumor Development, Immunology and Progression Laboratory is focused on an innovative research approach to improving outcomes for patients with pancreatic cancer.
Both KRas-activating mutations and oxygen radicals, such as superoxide, have important roles in mediating pancreatic cancer, most likely by signaling through activation of NF-κB. Moreover, protein kinase D (PKD), NF-κB-inducing kinase (NIK) and other kinases that activate NF-κB signaling are upregulated in pancreatic ductal adenocarcinoma and are potential drug targets.
Other inducers of NF-κB in pancreatic cancer cells are inflammatory cytokines, such as TNFα, that are released by macrophages, or mutant KRas-caused engagement of the epidermal growth factor receptor (EGFR).
Dr. Storz and his research team hope to determine the roles of KRas- and NF-κB-induced signaling pathways in the development and progression of pancreatic cancer and to understand the crosstalk of such signaling with cells of the innate immune system.
Dr. Storz ultimately hopes to develop both tools and methods for early detection of pancreatic cancer and to identify new targets for therapy.
His lab's research on signaling events underlying the development and progression of pancreatic cancer has three main focus areas:
1. Acinar-to-ductal metaplasia and PanIN formation as a cause of pancreatic disease
After tissue injury, inflammation or expression of an oncogenic mutation of KRas, pancreatic acinar cells undergo reprogramming that induces their transdifferentiation to a premature, ductlike phenotype. Cells that undergo acinar-to-ductal metaplasia can progress to develop pancreatic intraepithelial neoplasia (PanIN) and eventually pancreatic cancer.
The current focus of the Tumor Development, Immunology and Progression Laboratory is on understanding the mechanisms that drive such reprogramming of primary pancreatic acinar cells. A specific focus is on the crosstalk between cells of the innate immune system and pancreatic cells and on KRas-initiated signaling pathways.
Understanding such processes may lead to the development of early detection methods for pancreatic ductal adenocarcinoma (PDA) or novel treatment methods.
The lab uses transgene and knockout animal models, 3-D explant organoid cultures of primary acinar cells, and viral delivery systems to support this research. Data can be correlated to patient tissue microarrays generated in the laboratory.
2. Reactive oxygen species in development and progression of pancreatic cancer
Reactive oxygen species are generated in response to metabolic changes, mitochondrial dysfunction and many stressors, and increased occurrence of oxidative stress is a common phenotype in cancer.
Extensive work in Dr. Storz's laboratory has provided evidence for a previously undescribed signaling pathway that is activated by oxidative stress.
Dr. Storz and his team have shown that in cancer cells, when cellular reactive oxygen species (ROS) increase, protein kinase D (PKD) localizes to the mitochondria, where it is activated. Active PKD then signals to the nucleus via induction of the transcription factor NF-κB. The lab team has confirmed activation of this signaling pathway by oncogenic KRas as a key event driving the development of pancreatic cancer.
The lab's most recent projects focus on the mechanism of how protein kinase D (PKD) is targeted to the mitochondria, on understanding the mechanisms of how PKD1 activates ROS-regulated transcription factors, and on determining their target genes.
Another focus is to determine if this pathway can be targeted in pancreatic cancer using PKD inhibitors, either alone or in combination with currently used chemotherapy, in genetic (KC or KPC) animal models, or in orthotopic (cell lines or patient-derived xenografts) animal models.
3. Macrophage populations in initiation and progression of pancreatic cancer
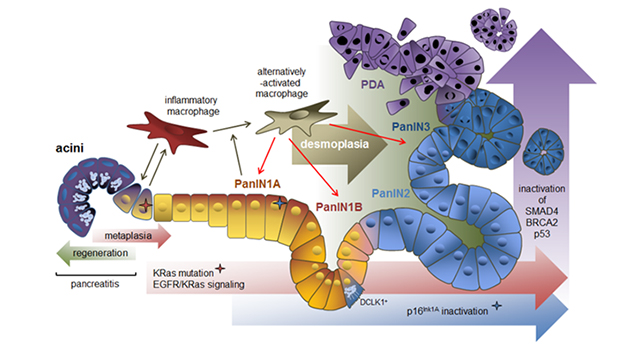
This schematic image shows how macrophage subtypes and genetic mutations contribute to acinar-to-ductal metaplasia (ADM), clonal expansion and progression to pancreatic cancer. ADM is a reversible process during pancreatitis, but it becomes irreversible when an oncogenic KRas mutation is present. The accumulation of KRas activity and loss of senescence due to inactivation of cyclin-dependent kinase inhibitor 2A (CDKN2A, p16INK1A) is needed for progression to PanIN1. Further progression to PanIN2, carcinoma in situ (PanIN3) and pancreatic ductal adenocarcinoma occurs after acquisition of additional gene mutations in TP53 (p53), BRCA2 and SMAD4. The progression to cancerous lesions occurs with an increase in desmoplasia. Cells positive for the kinase DCLK1 are of acinar origin, are formed mainly in low-grade PanIN lesions and have cancer stem cell functions.
In a series of scientific papers, Dr. Storz and his lab team showed that inflammatory macrophages can drive the initiation of pancreatic cancer. For example, the researchers found that macrophages can cause acinar-to-ductal metaplasia (ADM) by secreting inflammatory cytokines such as TNF and RANTES.
In addition, the lab found that after acquisition of an oncogenic KRas mutation, acinar cells start expressing chemoattractants for inflammatory macrophages. The presence of oncogenic KRas and inflammatory macrophages then drive the progression of ADM to PanIN lesions.
The lab's recent work focuses on how PanIN cells can crosstalk to inflammatory macrophages. Dr. Storz and his team found that tuft cells in panIN lesions secrete IL-13 to initiate a macrophage switch to an alternatively activated phenotype.
The lab's current focus is on developing strategies to revert this alternatively activated phenotype to an inflammatory macrophage phenotype.