-
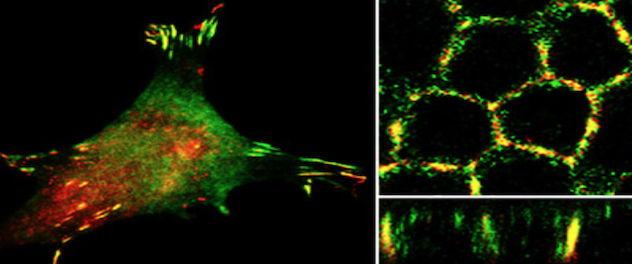
Cellular processes regulated by phosphoinositide signaling
Dr. Ling's lab seeks to define the role of phosphoinositide signaling in cell migration, epithelial morphogenesis, and the assembly and function of primary cilia by modulating protein-protein and protein-membrane interactions in the context of development, and diseases such as cancer metastasis and ciliopathies.
-
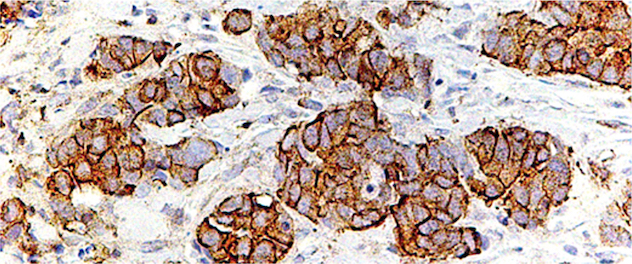
Cellular processes regulated by phosphoinositide signaling
Dr. Ling's lab seeks to define the role of phosphoinositide signaling in cell migration, epithelial morphogenesis, and the assembly and function of primary cilia by modulating protein-protein and protein-membrane interactions in the context of development, and diseases such as cancer metastasis and ciliopathies.
-
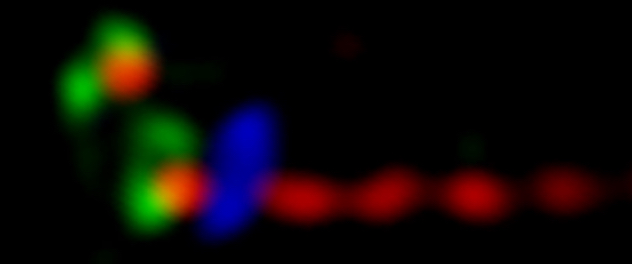
Cellular processes regulated by phosphoinositide signaling
Dr. Ling's lab seeks to define the role of phosphoinositide signaling in cell migration, epithelial morphogenesis, and the assembly and function of primary cilia by modulating protein-protein and protein-membrane interactions in the context of development, and diseases such as cancer metastasis and ciliopathies.
-
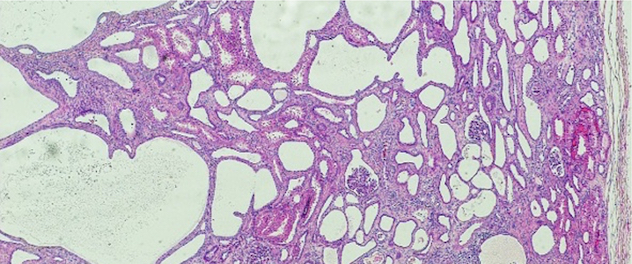
Cellular processes regulated by phosphoinositide signaling
Dr. Ling's lab seeks to define the role of phosphoinositide signaling in cell migration, epithelial morphogenesis, and the assembly and function of primary cilia by modulating protein-protein and protein-membrane interactions in the context of development, and diseases such as cancer metastasis and ciliopathies.
Overview
Phosphoinositides are reversibly phosphorylated derivatives of the membrane phosphatidylinositol (PtdIns). Phosphoinositides consist of two fatty acid chains, a glycerol moiety, and a D-myo-inositol-1-phosphate head group that is decorated by phosphate moiety at the 3-, 4-, or 5-hydroxyl position to produce seven phosphorylated derivatives:
- PtdIns(3)P
- PtdIns(4)P
- PtdIns(5)P
- PtdIns(3,4)P2
- PtdIns(3,5)P2
- PtdIns(4,5)P2
- PtdIns(3,4,5)P3
The exact composition of each species varies in different subcellular locations and different cell types. Compared with other phospholipids in cellular membrane compartments, phosphoinositides are relatively minor constituents. PtdIns(4)P and PtdIns(4,5)P2, which represent the most abundant phosphoinositide species, are less than 1% of the total cellular phospholipid pool. Despite the low abundance, phosphoinositides are indispensable to the integrity of cells. Phosphoinositides play essential roles in various cellular events including, but not limited to:
- Cytoskeletal dynamics.
- Cell adhesion and migration.
- Vesicular trafficking.
- Assembly of cargo proteins at membranes, cell proliferation, and survival.
Importantly, phosphoinositides are key identity determinants of various cellular membrane compartments and act as spatiotemporal cues to direct signaling events.
Each phosphoinositide species exhibits unique distribution across the different plasma membrane and organelle membrane compartments. The lipid tail of phosphoinositides inserts into membrane compartments. Whereas the phosphorylated inositol head of individual phosphoinositides recognizes and binds to unique phosphoinositide effector proteins. Current reported phosphoinositide-binding domains include:
- PH
- FYVE
- PX
- C2
- PROPPIN
- PTB
- Tubby
- TRAF
- ANTH/ENTH
- FERM
- PDZ
The affinity and specificity vary between different phosphoinositides and binding domains. Individual phosphoinositide species cause effector proteins to change their shapes and may also recruit them to specific subcellular locales to meet their signaling partners. This leads to the change of activity and/or availability of phosphoinositide effectors at specific subcellular locales. Thus, the precisely controlled spatiotemporal availability of phosphoinositide species in the cell is critical for the dynamics of relative cellular events.
Phosphoinositide metabolism and homeostasis are maintained and regulated by specific phosphoinositide kinases and phosphatases that are conserved in evolution. Many reports have discovered that the dysfunction of components of the phosphoinositide signaling pathway is responsible for various human diseases. These range from rare genetic diseases to more common conditions such as cancer, neurological disorders, cardiovascular disease, and metabolic disorders including obesity and diabetes.
The Phospholipid Signaling in Embryonic Development and Human Diseases Lab is interested in defining how specific phosphoinositide species, enzymes and effectors coordinate precisely in a spatiotemporal manner to achieve the regulation of relative cellular events, tissue homeostasis, and organ development and functions.
About Dr. Ling
Kun Ling, Ph.D., is a professor of biochemistry and molecular biology at Mayo Clinic College of Medicine and Science in Rochester, Minnesota. Dr. Ling's research focuses on phosphoinositide signaling and its role in various cellular processes and human diseases.