Projects
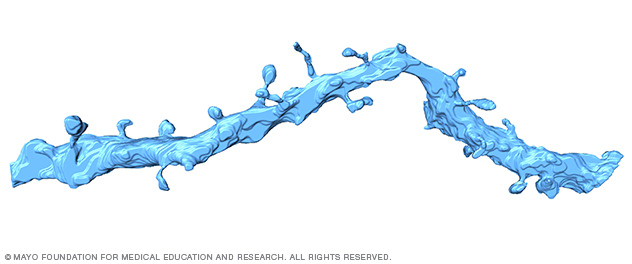 3D neuronal dendrite in the brain
3D neuronal dendrite in the brain
This high-resolution, 3D image has been reconstructed from a prefrontal rodent brain using a serial block-face electron microscope (SBEM) and Amira software.
Genetic dissection of neuroadaptation in the striatum and alcoholism
A neuroadaptative process within the glutamate-mediated signaling system comprises a critical consequence of alcoholism in humans. Since adenosine levels regulate glutamate signaling in the striatum, which may contribute to some aspects of alcoholism, deciphering gene expression at the cellular and circuitry levels of the striatum is crucial to understand ethanol intoxication and preference as well as to identify molecular signatures of clinically available medications such as acamprosate and topiramate. Furthermore, molecular changes in response to these clinically available drugs could lead to the identification of novel biomarkers or endo-phenotypes.
Studies with mice lacking ethanol-sensitive adenosine transporter (ENT1) displayed human alcoholic-like behaviors, which appear to be attributed to increased glutamate signaling in the striatum. Based on our recent proteomic and functional proteomic studies, we are aiming to understand whether:
- Two different striatal neurons (striatopallidal and striatonigral neurons) respond to ethanol differentially depending on ethanol doses and duration of exposure
- Clinically available acamprosate and topiramate alter ethanol-sensitive gene expression
- Novel drugable targets could be clinically useful
Our research will advance molecular understanding of alcoholism at anatomical and circuital levels, as well as provide an integrated tool to identify and validate novel candidate targets to treat alcohol use disorders, and could be applied to other behavioral and neuropsychiatric disorders.
Alcohol and adenosine-mediated glutamate signaling in neuro-glial interaction
Studies with ENT1 null mice indicate that ENT1 regulates ethanol intoxication and preference, as well as motivational effects of ethanol. These behaviors appear to be attributed to elevated glutamate signaling in the striatum where cortical glutamatergic axons mainly send their signals to control motor functions, habits and motivations. We found that EAAT2 expression was reduced in ENT1 null mice, indicating that the increased glutamate levels in ENT1 null mice may be partly due to the reduced synaptic glutamate uptake by EAAT2 in astrocytes.
Despite evidence demonstrating that a genetic variant of EAAT2 (G603A) is implicated in alcoholism, regulation of astrocytic EATT2 expression and function in response to ethanol is poorly understood at molecular, cellular and behavioral levels. Our laboratory focuses on identifying a novel ENT1 and EAAT2 signaling pathway that regulates ethanol responses in astrocytes and neuro-glial interactions, which may contain targets for the development of new therapeutics to treat alcohol use disorders in humans.
Translating alcoholism toward individualized treatment: Functional genomics and neuroimaging
Our recent findings show that the nonsynonymous 647T/C variant of ENT1 that replaces isoleucine with threonine at transmembrane domain 6 is associated with higher predisposition to alcohol withdrawal seizures in people with alcoholism. Functional analysis demonstrated that prolonged ethanol exposure increased uptake activity of ENT1-216Thr both for inosine and adenosine as compared to ENT1-216Ile. Our findings reveal an important role of a genetic variant of ENT1 in developing alcohol withdrawal-induced seizures in humans and animal models.
Our laboratory currently is examining glutamate and other brain metabolites such as glutamate, N-acetylaspartate (NAA), choline, inositol compounds and total creatine in both the anterior cingulated (AC) and the ventral striatum (NAc) and found that the levels are altered when comparing the basal level to that during an alcohol withdrawal period using in vivo 7T (300 MHz) and 16.5T (700 MHz) MRS (magnetic resonance spectroscopy). Collaborating with clinical investigators at Mayo Clinic, we are examining glutamate and other brain metabolites in human subjects as well.
We believe that our research investigating genetic predispositions and neuroimaging profiles is critically important to advance alcoholism treatment as well as other neuropsychiatric disorders.