Uncovering Signaling Pathways That Regulate Invasion and Metastasis of Cancer Cells
Current statistics for breast cancer show that survival could be significantly increased with better and earlier diagnosis of patients who are at risk of malignant progression.
Cellular mechanisms that contribute to early metastatic events are the reorganization of the actin cytoskeleton and the epithelial to mesenchymal transition (EMT).
Research from Dr. Storz's lab indicates that protein kinase D (PKD) enzymes regulate both of these key events. Moreover, the expression of different PKD isoforms seems to lead to different outcomes. While PKD1 mediates cell proliferation and also acts as a suppressor of breast tumor cell invasion, PKD3, when expressed, mediates all oncogenic functions.
The lab's ongoing research on breast cancer focuses on how different PKD enzymes regulate cell motility and invasiveness and how this knowledge can be translated to develop strategies and tools for early detection and treatment of metastatic breast cancer.
The lab is investigating several areas of research related to breast cancer:
1. Identification of cellular mechanisms regulating tumor cell motility
Directed tumor cell migration is regulated by processes that are controlled by RhoGTPases and lead to membrane protrusion at the leading edge. In response to chemotactic stimuli, this is mediated by enzymes that facilitate severing of F-actin structures, nucleation and branching of new F-actin filaments, and capping of filaments.
Dr. Storz's lab found that RhoA inhibits directed cell migration via activation of PKD1. The lab's work shows that PKD1 is a key enzyme regulating ADF/cofilin-mediated barbed end formation, actin incorporation and directed cell migration. Dr. Storz and his team have identified and characterized a multitude of PKD1 substrates that contribute to the regulation of cell motility, including PAK4, SSH1L and VASP.
In addition, the lab recently found that PKD1 also regulates focal adhesion dynamics by phosphorylating PIP5K1γ. The net effect of PKD1-mediated phosphorylation of these substrates is the global inhibition of actin reorganization, explaining the dramatic effects of PKD1 on cell migration.
Using a unique tool, an antibody that recognizes phosphorylated PKD substrates, the lab has identified additional targets for protein kinase D (PKD). Current research is exploring how phosphorylation by PKD1 alters their function with respect to actin reorganization and cell motility.
2. Identification of cellular mechanisms regulating epithelial to mesenchymal transition
Gain of function or reverse genetics cell culture experiments and in vitro 3-D cell culture (3-D acini model) indicated that loss of PKD1 is crucial for breast cancer cell invasion.
One of the functions that the lab has identified for PKD1 in normal breast tissue is that it maintains the epithelial phenotype of cells by inhibiting TGFβ- and Snail-induced epithelial-to-mesenchymal transition. Work in the lab also showed that PKD1 is a suppressor of several pro-invasive matrix metalloproteinases and that PKD1 regulates E-cadherin localization and signaling. The lab is also investigating other mechanisms of how PKD1 may regulate and maintain the epithelial phenotype.
3. Protein kinase D enzymes as markers and targets for invasive breast cancer
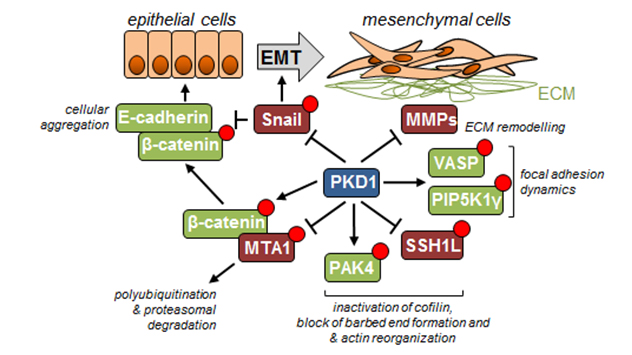
This schematic image shows some of the key events regulated by PKD1 and preventing EMT and decreasing cell motility. PKD1 blocks actin cytoskeleton rearrangements needed for cell movement through phosphorylation of SSH1L (inactivation) and PAK4 (activation), with a net effect of inhibiting cofilin. PKD1 also affects filopodia formation and length through phosphorylation of VASP, as well as focal adhesion dynamics by targeting PIP5K1γ and VASP. EMT and remodeling of extracellular matrix are regulated through phosphorylation and proteasomal degradation of Snail and by inhibition of MMP expression. In addition to increasing E-cadherin expression through inactivation of Snail, PKD1 also regulates the formation of E-cadherin-mediated cell-cell connections through phosphorylation of MTA1 and β-catenin, leading to MTA1 proteasomal degradation and β-catenin location to the E-cadherin complexes.
Given the current statistics in breast cancer for women living in Western nations, it is well accepted that survival could be significantly increased with better and earlier diagnosis of patients who are at risk of malignant progression.
With PKD1, Dr. Storz's lab has identified a kinase that is epigenetically downregulated in its expression in approximately 90 percent of all analyzed invasive human breast cancer samples compared with normal epithelial breast tissue. In contrast to downregulation of PKD1, the lab found that invasive breast cancers increase expression of PKD3, a bona fide oncogenic version of this kinase family.
Current research in the laboratory focuses on developing assays that can be clinically used to determine PKD1 and PKD3 expression in patient samples. The lab's goal in this work is to identify patients with an expression pattern that would recommend treatment with PKD inhibitors, either alone or in combination treatment with currently used drugs.