The Electrogenic Na+/HCO3- Cotransporter, NBC
A major task of the proximal tubule (PT) is to reabsorb NaHCO3 while maintaining intracellular pH (pHi). Filtered HCO3– is reclaimed in a three-step process (Fig 1A): (i) H+ is secreted into the lumen by Na-H exchangers and H+ pumps, titrating HCO3– into CO2 + H2O. (ii) This CO2 and H2O enter the cell, regenerating H+ + HCO3–. (iii) This HCO3– exits across the basolateral membrane (BLM). All told, the PT reabsorbs some 80-90% of filtered HCO3–, and 70% of the filtered Na+. The electrogenic Na/HCO3 cotransporter carries almost all of this HCO3–, and ~7% of the Na+. The renal electrogenic Na/HCO3 cotransporter moves HCO3– out of the cell and is thought to have a Na+:HCO3– stoichiometry of 1:3.
We cloned electrogenic Na+/HCO3- cotransporter(NBC1) from the Ambystoma tigrinum kidney using the expression cloning technique (Romero et al. Nature 487:409-413, 1997). Subsequently, we have cloned several other species variants and isoforms of NBC1. We have also developed several NBC-specific antibodies for localizing NBC in native tissue (Schmitt et al. Am. J. Physiol. 276: F2736, 1999). As indicated above, NBC1 is localized to the basolateral membrane of the renal proximal tubule. NBC1 mRNA and protein are somewhat widespread, found in brain, eye, heart, intestine, lung, liver, pancreas, stomach, etc. (Romero and Boron, Ann. Rev. Physiol. 61: 699-723, 1999; Romero, Journal of the Pancreas (online) 2: 182-191, 2001).
There are 3 NBC1 isoforms, i.e, mRNAs transcribed from the same gene (see Figure to the left). The original NBC1 clones were from the kidney and encoded the kNBC1 or "short" NBC protein. This NBC1 isoform while found as minor components in other tissues, is by far the dominant NBC1 isoform in the kidney. The longer N-terminal variant, pNBC1, seems to be the more general protein form.
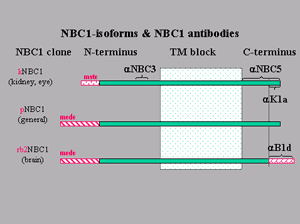
Schematic of electrogenic Na+/HCO–3 cotransporter isoforms and antibodies
Diagram of the three NBC1 isoforms: two N-terminal (kNBC1 and pNBC1) and one additional C-terminal (rb2NBC1). All other sequence between clones is identical within a species. Sequence regions recognized by NBC1 antibodies are indicated by brackets and annotated with "a." Modified from Romero, JOP 2: 182-191, 2001.
The human NBC1 gene (SLC4A4) resides at 4q21 (Abuladze et al. J Biol Chem 273: 17689-95, 1998; Romero et al, J. Am. Soc. Nephrol. 9: 11A, 1998). Recent data indicates that SLC4A4 is ~400-450 kb (Abuladze et al. Gene 251: 109-22, 2000). Both pNBC1 and kNBC1 are transcribed from the same gene; kNBC is apparently transcribed from an alternative internal promoter (Abuladze et al. Gene 251: 109-22, 2000).
In recent physiologic studies, we explored the cation and voltage dependence of NBC1. We were surprise to find that the "renal" NBC1 (kNBC1) has a ion-coupling (stoichiometry) of 1 Na+: 2 HCO3– rather than 1:3 as previously thought (Sciortino and Romero, Am. J. Physiol., 277: 611-623, 1999). Our present hypothesis is that there are specific binding / interacting proteins in the proximal nephron that alter the stoichiometry of NBC.
Since NBC1 cotransports Na+ and HCO3– ions, we hypothesized that mutations in NBC1 or alterations of NBC1 function might be manifest particularly in the kidney as a proximal renal tubular acidosis and / or a type of hypertension (Romero et al. Nature 387:409-13, 1997; Romero and Boron, Ann. Rev. Physiol. 61: 699-723, 1999). In fact, work of Igarashi and colleagues confirmed part of this hypothesis. These investigators found 2 families with homozygous NBC1 mutations (R298S and R510H) that present with severe proximal renal tubule acidosis and several ocular abnormalities, including bilateral glaucoma, bilateral cataracts, and bandkeratopathy (Igarashi et al., Nat Genet 23: 264-6, 1999).
Using site-directed mutagenesis, we have recreated these point mutations in the human kidney NBC1 clone (wild-type = hkNBC1). Our present studies with R510H indicate that the R510H-hkNBC1 protein is not efficiently trafficked to the plasma membrane as expressed in Xenopus oocytes (Sciortino and Romero. FASEB J 15: (4) A502, Part 1 MAR 7 2001). It is not clear whether this is an inherent protein processing problem or whether some other factor is present / absent in the oocyte system.