-

Discovering new therapeutic treatments for IBD
Dr. Faubion's lab seeks to discover, identify and develop individualized treatments to improve treatment options for people with inflammatory bowel disease (IBD).
-
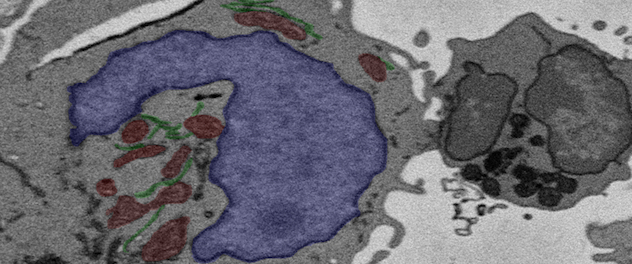
Characterizing the epigenetics of T-cell fate decisions
Understanding the regulatory mechanisms necessary for T-regulatory (Treg) gene expression will help us improve T-regulatory suppression in the setting of intestinal inflammation.
-
Understanding inflammatory and epigenetic mechanisms driving IBD pathophysiology
Dr. Faubion's Lab uses colitis mouse models and conditional knockout models to understand the loss of immunosuppressive function in T-regulatory cells.
-
Building an IBD biobank to discover molecular signatures
Clinical samples are a critical component in our research to help discover new therapeutic targets by examining the molecular signatures responsible for clinical phenotype. Dr. Faubion's team has an active biobank of IBD samples for analysis using discovery platforms.
-
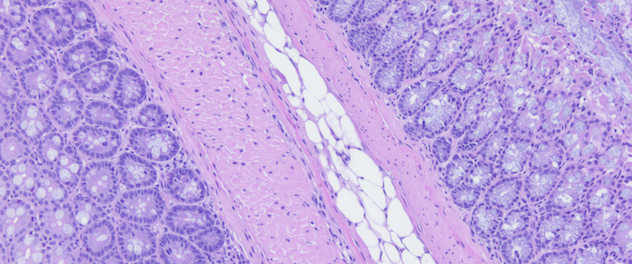
Using a systems biology approach to study IBD pathophysiology
Using stool, biopsies, serum and immune cells, the lab takes a systems biology approach toward understanding the complex nature of how molecular networks influence each other to drive intestinal inflammation.
Overview
Research in the Immuno-Epigenetics Laboratory, led by Dr. William A. Faubion Jr., is focused on understanding and identifying the epigenetic landscape of immune cells. The group's perspective is that the immune system fails to regulate inflammation chronically manifested in the autoimmune inflammatory bowel diseases (IBDs), such as Crohn's disease (CD) and ulcerative colitis (UC).
Despite some genetic variation associated with IBD, environmental factors also have been shown to increase the risk of developing IBD. Epigenetics is the study of the heritable changes to gene expression without changing the DNA code. Environmental signals may influence the dynamics of these epigenetic changes leading to an inheritable alteration of gene expression. Thus, the overall goal of the lab is to understand how environmental factors influence immune cellular development and function through chromatin remodeling.
Dr. Faubion's lab has previously found polycomb repressive complexes (PRCs) to be important in maintaining T-regulatory development and function. Loss of these complexes leads to severe colitis phenotype in mouse models and impaired regulatory function in in vitro experiments. His team also found forkhead box protein 3 (FOXP3) and enhancer of zeste homolog 2 (EZH2), a component of polycomb repressive complex 2 (PRC2), to be associated with deregulated pathways in CD4+ T cells isolated from lesions in people with Crohn's disease. This finding has propelled the current research projects into other epigenetic repressive complexes and cytokine-receptor signaling.
Research focus areas
Currently, the lab's goals include:
- Dissection of the epigenetic mechanisms of development and function of Treg and T-helper 17 (TH17) cells.
- Discovery of novel molecular mechanisms of IBD through integration of high-dimensional data sets.
- Optimization of Treg cells ex vivo for adoptive cell therapy.
- Development of novel therapies, particularly addressing TH17 cellular development.
Dr. Faubion's lab uses molecular and biochemical techniques, as well as high-throughput sequencing to provide an unbiased approach to discover molecular mechanisms driving IBD. This comprehensive approach will help lead us toward the development of new therapeutic targets.
In addition to these techniques, the lab is investigating clinically relevant epigenetic inhibitors, long noncoding RNAs (lncRNAs) and CRISPR-Cas9 technologies to characterize and modify regulatory T cells. The group aims to use this information to develop and offer more effective clinical therapies for people.
Key research findings include:
About Dr. Faubion
William A. Faubion Jr., M.D., is the research and innovation chair for Mayo Clinic's Department of Medicine, and a professor of immunology, medicine and pediatrics at Mayo Clinic College of Medicine and Science in Rochester, Minnesota. Dr. Faubion also directs the Multidisciplinary Training in Digestive Diseases program and the Clinician-Investigator Training Program.