Mechanobiology
Dr. Farrugia's Cellular and Molecular Physiology of Gastrointestinal Disorders Laboratory studies the mechanosensitive ion channels that drive normal functions in the gastrointestinal tract. This work is in collaboration with the Gastrointestinal Mechanotransduction Laboratory, led by Arthur Beyder, M.D., Ph.D. The major focus of this work is to determine the mechanosensing mechanisms in the gut's smooth muscle cells. The lab hopes to develop new treatments for functional gastrointestinal diseases such as irritable bowel syndrome (IBS) and slow-transit constipation.
How is the gut's motor powered? The gastrointestinal tract has to be an effective pump for normal function. Like other pumps in the body, such as the blood vessels, heart and bladder, the gastrointestinal tract uses transmembrane molecules called ion channels to generate electrical signals. The electrical activity generated by ion channels coordinates an influx of calcium required for smooth muscle cells to contract.
What do mechanosensitive ion channels have to do with it? Some ion channels are gated by specific primary stimuli such as voltage, but their function also is modulated significantly by other stimuli, such as ligands or force. In the gastrointestinal tract, where forces and electrical signals are critical for normal function, both channels primarily activated by force and those activated by voltage but modulated by force are especially relevant.
What techniques do we use? The lab uses conventional and advanced techniques to determine ion channel biophysics, and cellular, organ and in vivo physiology, related to ion channel function. We use conventional electrode-based electrophysiology, optogenetics, all-optical electrophysiology and ultrafast pressure clamps to study the molecular mechanisms of ion channel mechanosensitivity and its effects on gastrointestinal physiology and pathophysiology.
Channelopathies connect broken hearts with broken guts. Ion channels are expressed everywhere, and often in multiple organs at the same time. Ion channel mutations lead to arrhythmias in the heart and seizures in the brain, and Dr. Farrugia's team pioneered the work on channelopathies in the gut by improving understanding of how mutations affect smooth muscle physiology and gastrointestinal function overall.
In 2002, Dr. Farrugia's lab showed that NaV1.5, traditionally connected to the conduction system in the heart, is expressed in the human gut and that this voltage-gated ion channel is mechanosensitive. The lab's subsequent work dissected the mechanism of NaV1.5 mechanosensitivity at the molecular level and the relevance of NaV1.5 mechanosensitivity in gastrointestinal physiology and pathophysiology.
What are the biophysics of NaV1.5 mechanosensitivity? The lab found that there are specific NaV1.5 gating transitions that are affected by force and that in some cases, such as at the resting potential of the smooth muscle cell, this voltage-gated ion channel can be gated purely by force. Additionally, the lab found that NaV1.5 mechanosensitivity mechanism requires both the lipid bilayer and the cytoskeleton, and importantly that IBS-associated mutations alter NaV1.5 mechanosensitivity.
The lab then focused on the molecular mechanism of NaV1.5 mechanosensitivity. Ranolazine and lipid-soluble local anesthetics effectively inhibit NaV1.5 mechanosensitivity by a mechanism that is different from the mechanism these drugs use to block voltage sensitivity. The NaV1.5 mechanosensitivity blocked by ranolazine appeared to involve the lipid bilayer, and lipid-protein interactions in mechanosensitive ion channel function remains the lab's interest.
Colonic transit measured by scintigraphy shows a delay in colon transit in a patient with SCN5A mutation.
Colonic transit measured by scintigraphy shows a delay in colon transit in a patient with SCN5A mutation.
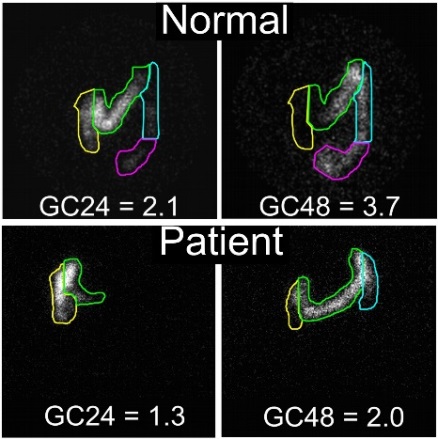
Colonic transit measured by scintigraphy shows a delay in colon transit in a patient with SCN5A mutation.
Top: Normal colonic transit. Labeled material is distributed among four colored sections, with an average position, or geometric center (GC), at 24 hours (GC24) being 2.1 and GC48 at 3.7. Bottom: Colonic transit in a patient with A997T-NaV1.5 mutation. The geometric center is significantly lower than normal for both GC24 (1.3) and GC48 (2.0). In this patient, the gastrointestinal transit was normalized by a treatment with a cardiac medication. Source: Gastroenterology. 2014;146:1659. Used with permission.
The lab's research is focused on why and how NaV1.5 is important for gastrointestinal physiology and pathophysiology. Dr. Farrugia's team has found that NaV1.5 mechanosensitivity is important for normal smooth muscle cell electrical and mechanical functions, and that a blockade of NaV1.5 mechanosensitivity by ranolazine decreases contractility, delays intestinal transit and results in constipation. Importantly, a subset of patients with IBS have SCN5A, a gene that codes for NaV1.5 mutations.
In this subset of patients, most of the mutations result in NaV1.5 channels that have functional abnormalities in both voltage-sensitivity and mechanosensitivity. However, Dr. Farrugia's team has found that restoring NaV1.5 function in these cases normalizes gastrointestinal function. This finding is significant in that NaV1.5 is the first case of ion channelopathy in functional gastrointestinal diseases.
In ongoing research, the lab aims to understand how IBS-associated SCN5A mutations affect smooth muscle physiology and gastrointestinal function overall, and how to pharmacologically target NaV1.5 mechanosensitivity in functional gastrointestinal diseases such as IBS and slow-transit constipation.
What is the future of gastrointestinal mechanobiology in Dr. Farrugia's Cellular and Molecular Physiology of Gastrointestinal Disorders Laboratory? The understanding of mechanobiology has trailed other fields due to a lack of dedicated tools and targets. This is rapidly changing. The 2021 Nobel Prize was awarded to Dr. Ardem Patapoutian for the discovery of the Piezo channels, which are bona fide mechanosensors. This work has begun to revolutionize this field.
Researchers studying mechanosensitive ion channels in Dr. Farrugia's lab work closely with the Gastrointestinal Mechanotransduction Laboratory of Arthur Beyder, M.D., Ph.D., at Mayo Clinic. Together, they seek to unravel the mysteries of the motor function and dysfunction in diseases such as IBS and slow transit constipation.
We seek to answer many outstanding questions in the field:
- What mechanogated and mechanosensitive ion channels are expressed in the gastrointestinal tract?
- What are the biophysical aspects of these channels' functions in the gastrointestinal tract?
- What are their functions in cells and tissues of the gastrointestinal tract?
- Are they misexpressed or mutated (channelopathies) in gastrointestinal motility disorders and disorders of gut-brain interaction?